Drop on Demand heading standard
New inline coding solution for pharmaceutical blister packs
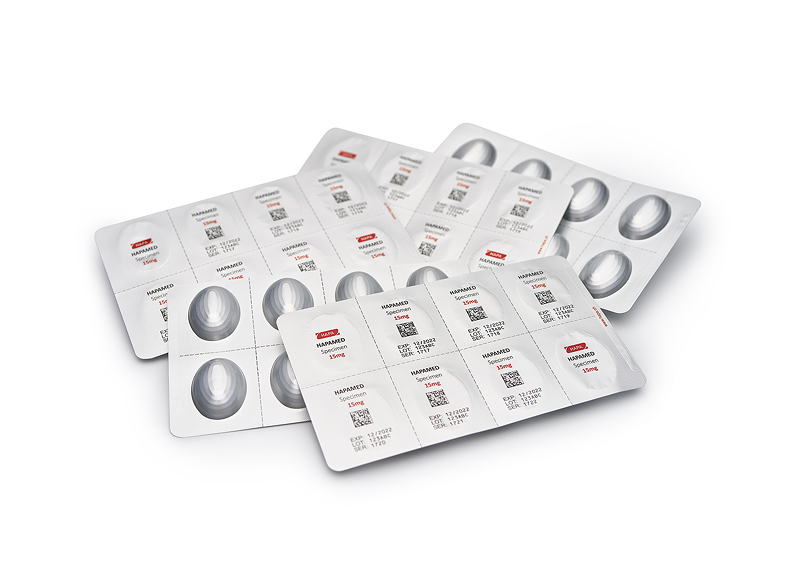
The variable data printer VDP 827 built by Hapa is capable of serializing or coding any part of the blister foil inline. It also supports all the standard symbologies and barcodes.
Quelle: Hapa
Despite having long set up times, having poor legibility and creating occasional rework, mechanical embossing remains the dominant technology for adding variable data on blister packs. In view of the adverse effect on both overall equipment effectiveness (OEE) and patient safety, pharmaceutical companies are urgently looking for solutions. With its new variable data printer VDP 827, Hapa is now proving how DOD inkjet technology – installed inline – makes marking and coding more efficient and reliable. Thanks to its highly compact design, the VDP 827 can be easily integrated into blister lines. The VDP concept was developed in consultation with the leading blister machine manufacturers. Since it is based on the proven Hapa Webjet technology, it is an accepted standard and details around mechanical and electrical integration have already been defined together with all key suppliers. For new blister line investments, the VDP represents an excellent technology choice.
“To achieve this level of standardization, we worked closely together with several manufacturers at the development stage, says Hapa sales director James MacKenzie. “Pharmaceutical companies thus attain printing processes that are optimally matched to the blister machines and benefit from standardized spare and wear parts that are available worldwide.”

The new VDP 827 is the first highly standardized inline DOD solution for variable data printing on pharmaceutical blister packs – and has already emerged as the first choice of practically all the major players in the blister line sector.
Quelle: Hapa
Built for diverse integration scenarios, the printer is also an ideal retrofit solution for converting existing blister lines to UV DOD printing.
Compared to traditional embossing, for example, the benefits of variable data printing with UV DOD technology are enormous: Razor-sharp 360 dpi print, no matter what the speed of the blister foil, enhanced camera readability and minimized waste. The VDP 827 can make full use of the web width of up to 288 mm and serialize or code any part of the blister foil. It also supports all the standard symbologies and barcodes. In addition, UV DOD printing can be read easily by people and vision systems alike.
UV DOD printing also outperforms other alternative technologies. Unlike ablative lasers, for example, the VDP 827 does not generate any dust or dirt whatsoever; and there’s no need for specially coated and therefore more expensive foils. No constraints apply in regards to format size or printing speed. As an entirely solvent-free technology, UV-curable DOD is also superior to thermal inkjet, according to a HAPA press release.
The UV cured ink is nonetheless available immediately for the next inline process step and is both extremely resilient and abrasion-proof. It can also be refilled on the fly without interrupting production – there is no need for tedious changing of cartridges, involving machine downtime. “The VDP 827 offers a simple and affordable pathway to the rewards of probably the most advanced inline printing technology for this particular application,” concludes James MacKenzie of Hapa. “It improves patient safety, reduces production complexity, cuts cost and increases OEE.”
Hapa AG is the global leader in late-stage customization and on-demand printing solutions for packaging processes in the pharmaceutical and healthcare industries.
Hapa, headquartered in Volketswil near Zurich, is part of Coesia, a group of companies specialised in highly innovative industrial and packaging solutions, headquartered in Bologna, Italy. The Group is present in 35 countries with 85 production facilities in 138 operating units and more than 8,000 employees.
(kb)